Description
GET THIS PRODUCT DELIVERED DOMESTICALLY WITHIN USA AT NOOTROPICSBASE.COM
CEREBROLYSIN® is a neuropeptide and hydrolysate of a porcine brain tissue developed in 1949 and first approved for medical use in 1954. Cerebrolysin in modern days is widely administered in 50 countries worldwide in Europe and Asia and used to improve the cognitive abilities by stimulating the regeneration of the nervous system. There is a vast array of studies (around 200) and human trials carried out in the past 15 years. The natural repair and recovery processes in the Central Nervous System (CNS) that start immediately upon injury and play an important role in the continuous defense against neurodegeneration in chronic CNS disorders (e.g. Alzheimer’s disease). Cerebrolysin® has shown to modify two major signalling pathways: the neurotrophic factor (NTF) and sonic hedgehog (Shh) signalling pathway. These pathways regulate on a molecular level the cellular processes of neurogenesis, angiogenesis, dendrite arborisation, axonal sprouting, myelination, and remodelling of the neurovascular unit, thereby supporting the maintenance and repair of the neuronal network.
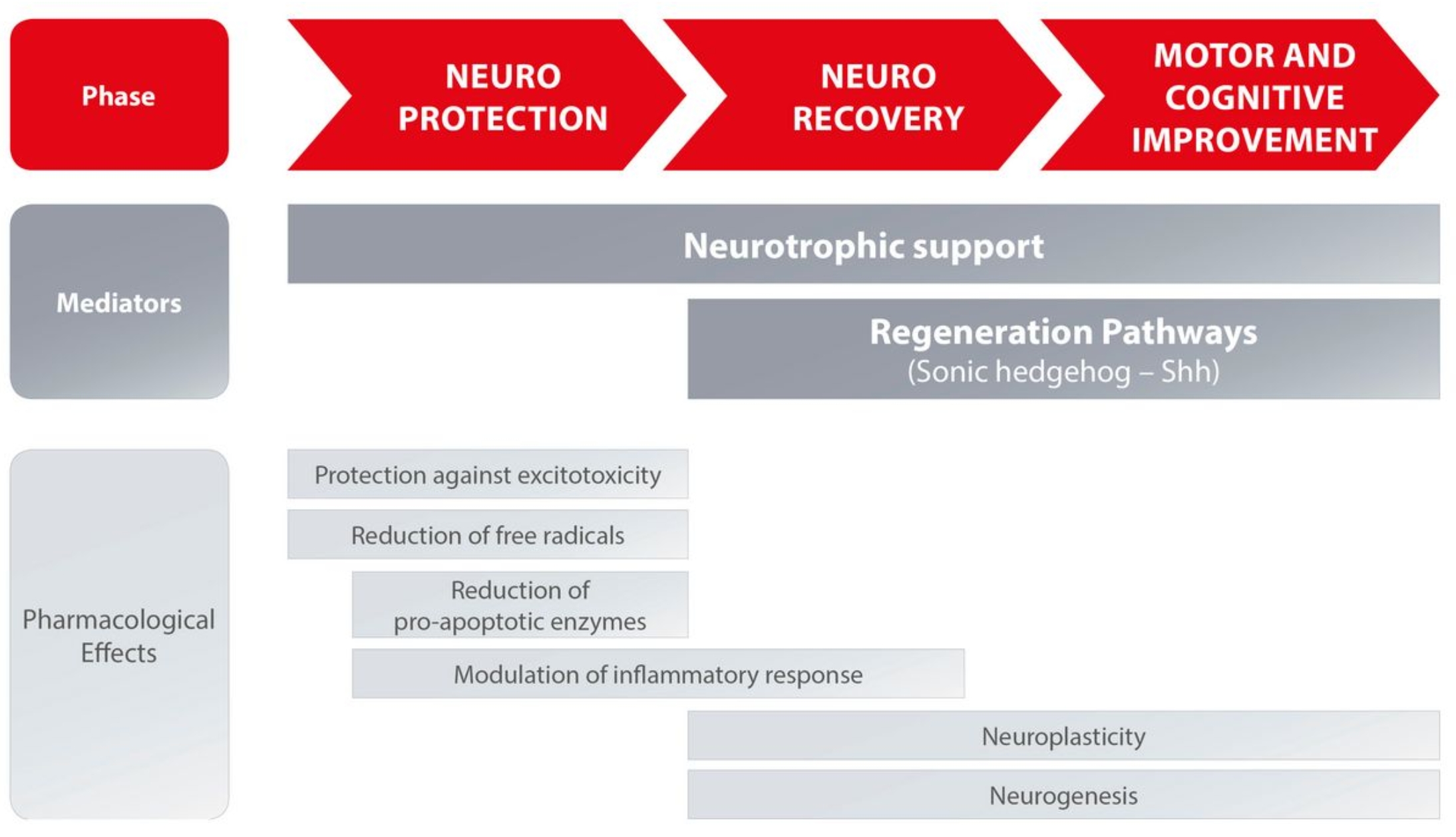
USES
- Treat Cerebrovascular Disorders.
- Prevent and Treat Senile Dementia of Alzheimer’s Type.
- Treat Vascular Dementia, Stroke.
- Treat Craniocerebral Trauma (Commotio and Contusio).
GUARANTEE & EXPIRY DATE
100% Original from the Austrian EverNeuro Pharma.
All packs are air tight sealed and expire up to 11.2025.
PACKAGING & DOSAGE
Active substance: cerebrolysin concentrate (complex of peptides obtained from the porcine brain tissue) 215,2 mg per 1 ml.
Excipients: sodium hydroxide, water for injection
Cerebrolysin is used as an injectable: intramuscular injection (2-5 ml), intravenous injection (2-10 ml) or intravenous drip infusion (10-50ml). The dose and duration depends on the nature and severity of the required treatment, as well as the patient's age. The maximum daily dose is 50 ml. Average adult daily dose is 10ml. Course of treatment is 10-20 days with daily injections.
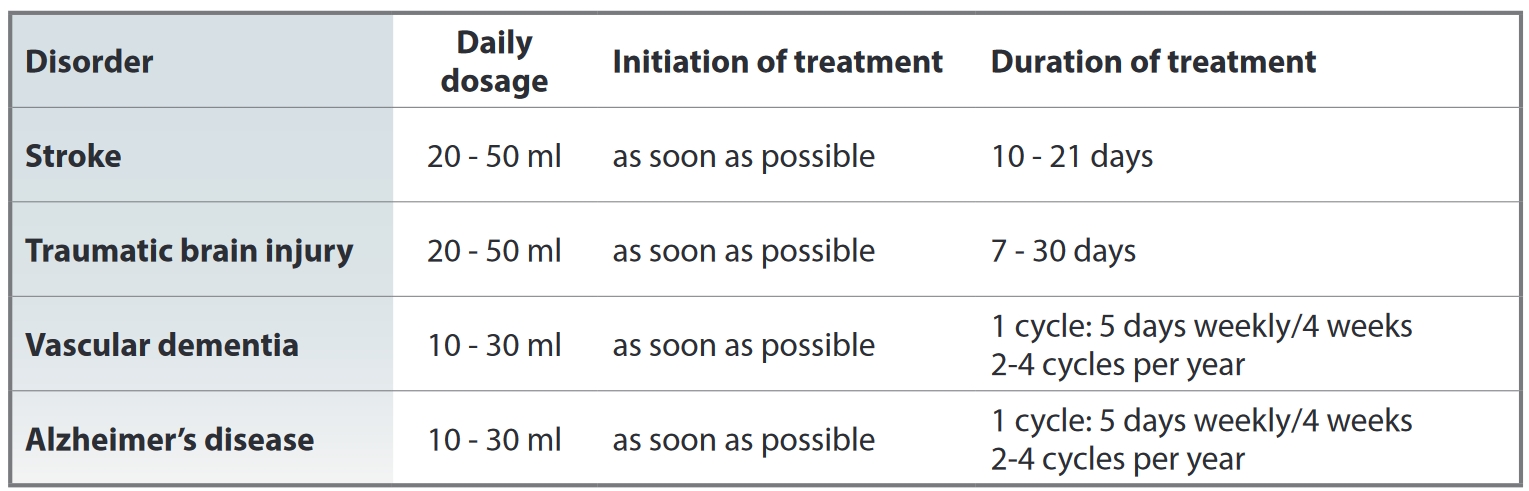
HOW TO ADMINISTER
1. Use only sterile, single use syringes (or single use one-way infusion kits). Recommended to use sterile plastic gloves.
2. Steralise the injection area with a alcohol prep wipe. See video guide for intramuscular injection sites.
2. Open ampoule with a new alcohol wipe.
3. Extract the contents of the ampoule immediately. Do not store an open ampoule.
4. A particle filter to filter out any broken glass is not required. The ampoules are specially made to break into large pieces only when opening.
6. Inject slowly.
7. Wipe down the injection area with an alcohol wipe.
8. Safely dispose of syringle, ampoule and wipes.
SIDE EFFECTS
The side effects of Cerebrolysin are rare and of mild intensity. The most frequently reported adverse reactions with Cerebrolysin are dizziness, headache, sweating, and nausea.
The MHRA and FDA has not evaluated or endorsed this product. Please consult your physician prior to using this or any other nutritional supplements or medications.
IS CEREBROLYSIN A SAFE DRUG?
Cerebrolysin has used for many years and the safety profile of the drug is well-established. Therefore the submission frequency for Periodic Safety Update Reports was set to 13 years by the European Medicines Agency (EMA).
FURTHER READ
Cerebrolysin: The basics by BodyHunter
Recovering from stroke Reddit review
Cerebrolysin 84 page PDF Monograph EverNeuro Pharma
STORAGE
Store in dry place at room temperature. Do not exceed storage temperature higher than 25 degrees Celcius. Do not freeze. Keep away from direct sunlight. Keep locked and away from children.
QUESTIONS?
Ask us any questions about CEREBROLYSIN.